Revisiting a classical redox process on a gold electrode by operando ToF-SIMS: where does the gold go?†
Abstract
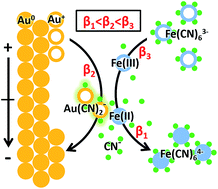
Electrochemical redox conversion between ferricyanide and ferrocyanide on a gold electrode is one of the most classical reactions in electrochemistry. In textbooks, the gold electrode is seen as chemically inert, on which only the adsorption/desorption of [Fe(CN)6]3/4− and electron transfer take place. Here, the electrochemical process of [Fe(CN)6]3/4− on a gold electrode was revisited using a vacuum-compatible microfluidic electrochemical cell in combination with operando liquid ToF-SIMS. An intermediate, Au(CN)2−, was observed in the cyclic voltammetry of ferricyanide with an interesting periodic potential-dependent variation trend. It was demonstrated that the gold electrode participated in the redox reaction of [Fe(CN)6]3/4− by competing with it to form Au(CN)2−, since the formation constant was Fe(CN)63− > Au(CN)2− > Fe(CN)64−. The formation and evolution of Au(CN)2− depends on the ratio of Fe(III) and Fe(II) on the surface of the gold electrode, which was determined by the redox conversion between Fe(III) and Fe(II) as well as the electric field force-based attraction or repulsion between the gold electrode and [Fe(CN)6]3/4−. Both of these factors were potential-dependent, resulting in the periodic change of Au(CN)2− in the dynamic potential scan of [Fe(CN)6]3/4−. These results provided solid molecular evidence for the participation of the gold electrode in the [Fe(CN)6]3/4− redox system, which will deepen mechanistic understandings of related electrochemical applications.


 Please wait while we load your content...
Please wait while we load your content...