Catalytic protodeboronation of pinacol boronic esters: formal anti-Markovnikov hydromethylation of alkenes†
Abstract
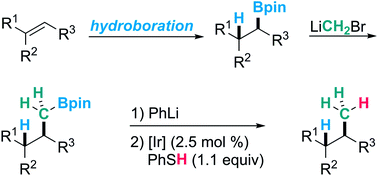
Pinacol boronic esters are highly valuable building blocks in organic synthesis. In contrast to the many protocols available on the functionalizing deboronation of alkyl boronic esters, protodeboronation is not well developed. Herein we report catalytic protodeboronation of 1°, 2° and 3° alkyl boronic esters utilizing a radical approach. Paired with a Matteson–CH2–homologation, our protocol allows for formal anti-Markovnikov alkene hydromethylation, a valuable but unknown transformation. The hydromethylation sequence was applied to methoxy protected (−)-Δ8-THC and cholesterol. The protodeboronation was further used in the formal total synthesis of δ-(R)-coniceine and indolizidine 209B.


 Please wait while we load your content...
Please wait while we load your content...