Synthesis of lamellarin alkaloids using orthoester-masked α-keto acids†
Abstract
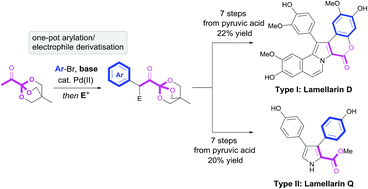
Pyruvic acid and other α-keto acids are frequently encountered as intermediates in metabolic pathways, yet their application in total synthesis has met with limited success. In this work, we present a bioinspired strategy that utilizes highly functionalized OBO (oxabicyclo[2.2.2]octyl) orthoester masked α-ketoacids as key intermediates for the construction of both type I and II lamellarin alkaloids. Lamellarin D was synthesized, via a key 1,4-dicarbonyl, in 7 steps and 22% yield from pyruvic acid. Key steps in the synthesis involve one-pot double enolate functionalisation of 1 followed by double annulation to form the target pyrrole/N-vinyl pyrrole core and late-stage direct C–H arylation. Lastly, a novel OBO-masked β-cyano ketone, synthesized from 1, proved to be a valuable intermediate for construction of the type II lamellarin core via HBr-mediated cyclisation. In this way, lamellarin Q was synthesized in 7 steps and 20% yield from pyruvic acid.


 Please wait while we load your content...
Please wait while we load your content...