A versatile catalyst system for enantioselective synthesis of 2-substituted 1,4-benzodioxanes†
Abstract
We report the synthesis of enantiomerically enriched 1,4-benzodioxanes containing alkyl, aryl, heteroaryl, and/or carbonyl substituents at the 2-position. The starting 1,4-benzodioxines were readily synthesized via ring closing metathesis using an efficient nitro-Grela catalyst at ppm levels. Excellent enantioselectivities of up to 99:1 er were obtained by using the versatile catalyst system [Ir(cod)Cl]2/BIDIME-dimer in the asymmetric hydrogenation of 2-substituted 1,4-benzodioxines. Furthermore, DFT calculations reveal that the selectivity of the process is controlled by the protonation step; and coordinating groups on the substrate may alter the interaction with the catalyst, resulting in a change in the facial selectivity.
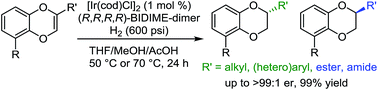


 Please wait while we load your content...
Please wait while we load your content...