Nickel-catalyzed oxidative C–H/N–H annulation of N-heteroaromatic compounds with alkynes†
Abstract
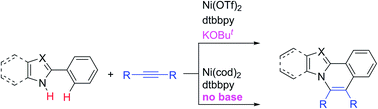
The reaction of N-heteroaromatic compounds, such as 2-aryl-pyrrole, benzimidazole, imidazole, indole, and pyrazole derivatives, with alkynes in the presence of a catalytic amount of a nickel complex results in C–H/N–H oxidative annulation. The reaction shows a high functional group compatibility. While both Ni(0) and Ni(II) complexes show a high catalytic activity, Ni(0) is proposed as a key catalytic species in the main catalytic cycle. In the case of the Ni(II) system, the presence of a catalytic amount of a strong base, such as KOBut, is required for the reaction to proceed. In sharp contrast, a base is not required in the case of the Ni(0) system. The proposed mechanism is supported by DFT studies.
- This article is part of the themed collection: Most popular 2019-2020 catalysis articles


 Please wait while we load your content...
Please wait while we load your content...