Mild complexation protocol for chiral CpxRh and Ir complexes suitable for in situ catalysis†
Abstract
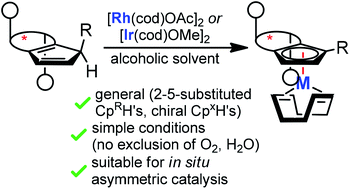
A practical complexation method for chiral cyclopentadienyl (Cpx) iridium and rhodium complexes is described. The procedure uses the free CpxH with stable and commercially available rhodium(I) and iridium(I) salts without base or additive. The conditions are mild and do not require the exclusion of air and moisture. A salient feature is the suitability for in situ complexations enhancing the user-friendliness of Cpx ligands in asymmetric catalysis. DFT-calculations confirm an intramolecular proton abstraction pathway by either the bound acetate or methoxide. Furthermore, the superior facial selectivity of the proton abstraction step enabled the development of TMS-containing trisubstituted Cpx ligands which display improved enantioselectivities for the benchmarking dihydroisoquinolone synthesis.
- This article is part of the themed collection: Celebrating our 2019 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...