Enantioselective synthesis of gem-disubstituted N-Boc diazaheterocycles via decarboxylative asymmetric allylic alkylation†
Abstract
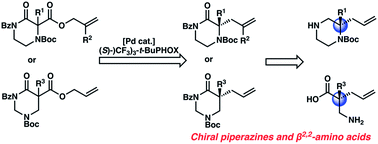
An enantioselective synthesis of diverse N4-Boc-protected α,α-disubstituted piperazin-2-ones using the palladium-catalyzed decarboxylative allylic alkylation reaction has been achieved. Using a chiral Pd-catalyst derived from an electron deficient PHOX ligand, chiral piperazinones are synthesized in high yields and enantioselectivity. The chiral piperazinone products can be deprotected and reduced to valuable gem-disubstituted piperazines. This reaction is further extended to enable the enantioselective synthesis of α,α-disubstituted tetrahydropyrimidin-2-ones, which are hydrolyzed into corresponding chiral β2,2-amino acids.


 Please wait while we load your content...
Please wait while we load your content...