Ba4Bi2(Si8−xB4+xO29) (x = 0.09): a new acentric metal borosilicate as a promising nonlinear optical material†‡
Abstract
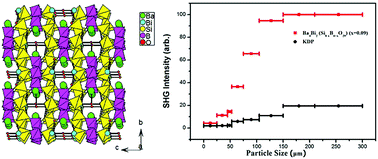
A new acentric metal borosilicate, namely Ba4Bi2(Si8−xB4+xO29) (x = 0.09), has been synthesized by a standard solid-state reaction. The title compound crystallizes in noncentrosymmetric (NCS) space group I![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2m with lattice parameters a = 11.0254(4) Å and c = 10.3961(9) Å. Structure refinements indicate that mixing of B atoms and Si atoms exists for a few atomic sites. In the “ideal” Ba4Bi2(Si8B4O29), BO4 or SiO4 tetrahedra are inter-connected by corner-sharing to cyclic B4O12 or Si4O12 units. These B4O12 and Si4O12 units are further interconnected via corner-sharing to an “ideal” [Si8B4O29]14− 3D network. The Ba2+ and Bi3+ act as the counter cations and are located at the cavities of the structure. Ba4Bi2(Si8−xB4+xO29) (x = 0.09) melts incongruently at a high temperature of 929 °C. Powder second-harmonic generation (SHG) measurements reveal that Ba4Bi2(Si8−xB4+xO29) (x = 0.09) is a type I phase-matching compound with a good SHG response of about 5.1 times that of KDP (KH2PO4), which is the highest among the borosilicates reported so far. The SHG source has been studied by DFT theoretical calculations. Our preliminary results indicate that Ba4Bi2(Si8−xB4+xO29) (x = 0.09) is a new second-order nonlinear-optical crystalline material candidate.
2m with lattice parameters a = 11.0254(4) Å and c = 10.3961(9) Å. Structure refinements indicate that mixing of B atoms and Si atoms exists for a few atomic sites. In the “ideal” Ba4Bi2(Si8B4O29), BO4 or SiO4 tetrahedra are inter-connected by corner-sharing to cyclic B4O12 or Si4O12 units. These B4O12 and Si4O12 units are further interconnected via corner-sharing to an “ideal” [Si8B4O29]14− 3D network. The Ba2+ and Bi3+ act as the counter cations and are located at the cavities of the structure. Ba4Bi2(Si8−xB4+xO29) (x = 0.09) melts incongruently at a high temperature of 929 °C. Powder second-harmonic generation (SHG) measurements reveal that Ba4Bi2(Si8−xB4+xO29) (x = 0.09) is a type I phase-matching compound with a good SHG response of about 5.1 times that of KDP (KH2PO4), which is the highest among the borosilicates reported so far. The SHG source has been studied by DFT theoretical calculations. Our preliminary results indicate that Ba4Bi2(Si8−xB4+xO29) (x = 0.09) is a new second-order nonlinear-optical crystalline material candidate.


 Please wait while we load your content...
Please wait while we load your content...