Crystallographic characterization of Lu2C2n (2n = 76–90): cluster selection by cage size†
Abstract
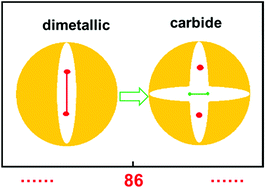
The successful isolation and unambiguous crystallographic assignment of a series of lutetium-containing endohedral metallofullerenes (EMFs), Lu2C2n (2n = 76, 78, 80, 84, 86, 88, 90), reveal an unrecognized decisive effect of the cage size on the configuration of the encapsulated clusters. The molecular structures of these compounds are unambiguously assigned as Lu2@Td(2)-C76, Lu2@D3h(5)-C78, Lu2@C2v(5)-C80, Lu2@C2v(7)-C84, Lu2@Cs(8)-C86, Lu2@Cs(15)-C86, Lu2@C1(26)-C88, Lu2C2@C2v(9)-C86, Lu2C2@Cs(32)-C88 and Lu2C2@D2(35)-C88. Specifically, when the cage is relatively small, Lu2@C2n (2n = 76–86) are all dimetallofullerenes (di-EMFs) and a Lu–Lu single bond could be formed between the two lutetium ions inside the cages. However, when the cage expands further, the valence electrons forming the possible Lu–Lu bond donate to a readily inserted C2-unit, resulting in the formation of carbide EMFs, Lu2C2@C2n (2n = 86, 88). Consistently, our theoretical results reveal that all these EMFs are thermodynamically favorable isomers. Thus the comprehensive characterization of the series of Lu2C76–90 isomers and the overall agreement between the experimental and theoretical results reveal for the first time that the exact configuration of the internal metallic cluster is determined by the cage size, taking a solid step towards the controlled synthesis of novel hybrid molecules which may have potential applications as building blocks of single molecule devices.


 Please wait while we load your content...
Please wait while we load your content...