Reduction of organic azides by indyl-anions. Isolation and reactivity studies of indium–nitrogen multiple bonds†
Abstract
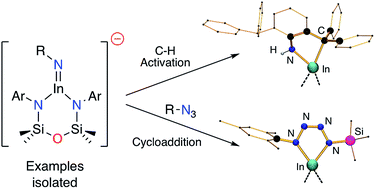
The synthesis of a new potassium–indyl complex, K[In(NONAr)] (NONAr = [O(SiMe2NAr)2]2−, Ar = 2,6-iPr2C6H3) and its reactivity with organic azides RN3 is reported. When R = 2,6-bis(diphenylmethyl)-4-tBu-phenyl, a dianionic alkyl-amide ligand is formed via C–H activation across a transient In–Nimide bond. Reducing the size of the R-group to 2,4,6-trimethylphenyl (mesityl, Mes) enables oxidation of the indium and elimination of dinitrogen to afford the imide species, K[In(NONAr)(NMes)]. The anion contains a short In–Nimide bond, shown computationally to contain appreciable multiple bond character. Reaction of isolated imides with an additional equivalent of azide (R = Mes, SiMe3) generates tetrazenido-indium compounds K[In(NONAr){κ-N,N′-N4(Mes)(R)-1,4}], shown by X-ray crystallography to contain planar InN4 heterocycles in the anion.


 Please wait while we load your content...
Please wait while we load your content...