δ C–H (hetero)arylation via Cu-catalyzed radical relay†
Abstract
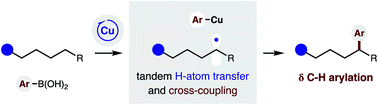
A Cu-catalyzed strategy has been developed that harnesses a radical relay mechanism to intercept a distal C-centered radical for C–C bond formation. This approach enables selective δ C–H (hetero)arylation of sulfonamides via intramolecular hydrogen atom transfer (HAT) by an N-centered radical. The radical relay is both initiated and terminated by a Cu catalyst, which enables incorporation of arenes and heteroarenes by cross-coupling with boronic acids. The broad scope and utility of this catalytic method for δ C–H arylation is shown, along with mechanistic probes for selectivity of the HAT mechanism. A catalytic, asymmetric variant is also presented, as well as a method for accessing 1,1-diaryl-pyrrolidines via iterative δ C–H functionalizations.
- This article is part of the themed collections: Most popular 2019-2020 organic chemistry articles, New reactivity in organic chemistry and Most popular 2018-2019 organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...