Biosynthesis of the RiPP trojan horse nucleotide antibiotic microcin C is directed by the N-formyl of the peptide precursor†
Abstract
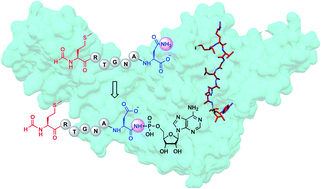
Microcin C7 (McC) is a peptide antibiotic modified by a linkage of the terminal isoAsn amide to AMP via a phosphoramidate bond. Post-translational modification on this ribosomally produced heptapeptide precursor is carried out by MccB, which consumes two equivalents of ATP to generate the N–P linkage. We demonstrate that MccB only efficiently processes the precursor heptapeptide that retains the N-formylated initiator Met (fMet). Binding studies and kinetic measurements evidence the role of the N-formyl moiety. Structural data show that the N-formyl peptide binding results in an ordering of residues in the MccB “crossover loop”, which dictates specificity in homologous ubiquitin activating enzymes. The N-formyl peptide exhibits substrate inhibition, and cannot be displaced from MccB by the desformyl counterpart. Such substrate inhibition may be a strategy to avert unwanted McC buildup and avert toxicity in the cytoplasm of producing organisms.


 Please wait while we load your content...
Please wait while we load your content...