Asymmetric maltose neopentyl glycol amphiphiles for a membrane protein study: effect of detergent asymmetricity on protein stability†
Abstract
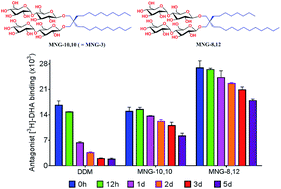
Maintaining protein stability in an aqueous solution is a prerequisite for protein structural and functional studies, but conventional detergents have increasingly showed limited ability to maintain protein integrity. A representative novel agent, maltose neopentyl glycol-3 (MNG-3), has recently substantially contributed to membrane protein structural studies. Motivated by the popular use of this novel agent, we prepared asymmetric versions of MNG-3 and evaluated these agents with several membrane proteins including two G protein-coupled receptors in this study. We found that some new MNGs were significantly more effective than MNG-3 at preserving protein integrity in the long term, suggesting that these asymmetric MNGs will find a wide use in membrane protein studies. In addition, this is the first study addressing the favorable effect of detergent asymmetric nature on membrane protein stability.


 Please wait while we load your content...
Please wait while we load your content...