A low-valent dinuclear ruthenium diazadiene complex catalyzes the oxidation of dihydrogen and reversible hydrogenation of quinones†
Abstract
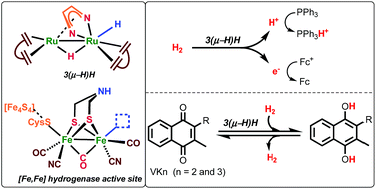
The dinuclear ruthenium complex [Ru2H(μ-H)(Me2dad)(dbcot)2] contains a 1,4-dimethyl-diazabuta-1,3-diene (Me2dad) as a non-innocent bridging ligand between the metal centers to give a [Ru2(Me2dad)] core. In addition, each ruthenium is bound to one dibenzo[a,e]cyclooctatetraene (dbcot) ligand. This Ru dimer converts H2 to protons and electrons. It also catalyzes reversibly under mild conditions the selective hydrogenation of vitamins K2 and K3 to their corresponding hydroquinone equivalents without affecting the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds. Mechanistic studies suggest that the [Ru2(Me2dad)] moiety, like hydrogenases, reacts with H2 and releases electrons and protons stepwise.
C double bonds. Mechanistic studies suggest that the [Ru2(Me2dad)] moiety, like hydrogenases, reacts with H2 and releases electrons and protons stepwise.


 Please wait while we load your content...
Please wait while we load your content...