Peroxidation of 2-oxindole and barbituric acid derivatives under batch and continuous flow using an eco-friendly ethyl acetate solvent†
Abstract
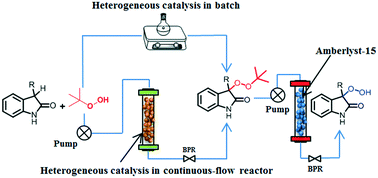
The C(sp3)–H peroxidation of 2-oxindole and barbituric acid derivatives using aliphatic peroxides under continuous flow and their antimalarial evaluation in vitro have been explored using magnetic iron oxide nanoparticles. This transformation uses less toxic, low cost, and eco-friendly ethyl acetate as a solvent. To show the robustness, supported catalysis integrated with continuous-flow was employed as a process development tool for the expeditious synthesis of quaternary peroxide derivatives with a residence time of 7.9 minutes. Additionally, the explosive hazards of TBHP (tert-butyl hydroperoxide) were also minimized during peroxidation in a continuous-flow process by controlled addition.


 Please wait while we load your content...
Please wait while we load your content...