Estimating speciation of aqueous ammonia solutions of ammonium bicarbonate: application of least squares methods to infrared spectra†
Abstract
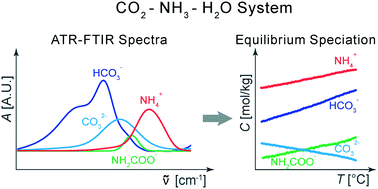
The knowledge of the speciation and of the supersaturation of aqueous solutions of CO2 and NH3 is pivotal for the design and optimization of unit operations, e.g. absorption or crystallization, in the framework of ammonia-based CO2 capture systems. For this information to be available, however, complex analytical techniques and significant experimental effort are required. This work introduces a methodology for the estimation of the concentration of species in aqueous ammonia solutions of ammonium bicarbonate by using attenuated total reflection infrared spectroscopy (ATR-FTIR) and spectral modeling based on least squares methods. In particular, the methodology can be exploited for the on-line monitoring of the liquid composition of crystallizing suspensions of ammonium bicarbonate for which the information on the speciation is combined with a rigorous thermodynamic model to compute the activity-based supersaturation. Finally, this work paves the way for the estimation of the crystallization kinetics of ammonium bicarbonate formation in aqueous ammonia solutions which is of great importance for the design of industrial CO2 capture absorption processes that exploit solid formation.


 Please wait while we load your content...
Please wait while we load your content...