Synthesis and thermally induced structural transformation of phthalimide and nitrile-functionalized benzoxazine: toward smart ortho-benzoxazine chemistry for low flammability thermosets†
Abstract
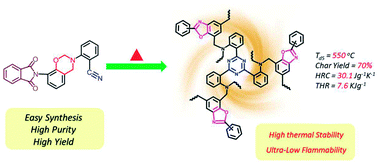
A novel ortho-phthalimide-functionalized benzoxazine monomer containing an ortho-nitrile group has been synthesized in order to further systematically evaluate the thermally induced structural transformation from benzoxazine resin to cross-linked polybenzoxazole. The chemical structure of the synthesized monomer has been confirmed by 1H and 13C nuclear magnetic resonance (NMR) spectroscopy and Fourier transform infrared (FT-IR) spectroscopy. Also supporting the detailed structure is 1H–13C heteronuclear multiple quantum coherence (HMQC), which identifies the local proton-carbon proximities. The polymerization behaviors, including the ring-opening polymerization of the oxazine rings and the cyclotrimerization of the nitrile functionalities, are studied by differential scanning calorimetry (DSC) and in situ FT-IR. In addition, the subsequent benzoxazole formation after polymerization has also been analyzed using thermogravimetric analysis (TGA) and magic-angle spinning (MAS) solid-state 13C NMR. The resulting cross-linked polybenzoxazole derived from the benzoxazine monomer exhibits exceptionally high thermal stability and low flammability, with an extremely high Td5 temperature (550 °C), a high char yield value (70%) and an extraordinarily low total heat release (THR of 7.6 kJ g−1).


 Please wait while we load your content...
Please wait while we load your content...