Core-modified 48π and 42π decaphyrins: syntheses, properties and structures†
Abstract
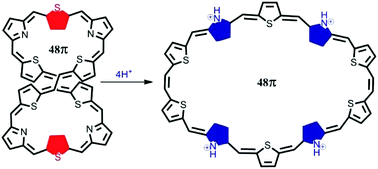
Syntheses, properties and structures of two decaphyrins containing 48π and 42π electronic circuits are reported. 48π decaphyrin 10 was obtained in 8% yield by MacDonald type acid catalysed condensation of appropriate precursors 6 and 7 followed by oxidation with DDQ. The product distribution and yield in this reaction depend on the concentration of trifluoroacetic acid (TFA) used. The 42π decaphyrin 12 was obtained in 12% yield by oxidative coupling reaction of 11 under acid catalysed conditions along with the formation of cyanated product 13. However in the absence of the acid catalyst, only 12 was isolated in 30% yield. Spectroscopic properties reveal that both 10 and 12 are nonaromatic in their freebase form and the single crystal X-ray structure of 10 indicates its figure-eight conformation. However, protonation of pyrrole nitrogens of 10 results in significant changes in the chemical shift of different ring protons with a large paratropic ring current indicating a transition from the nonaromatic state to the Hückel antiaromatic state. NICS(0) and HOMA values support the Hückel antiaromatic state of 10 on protonation. Protonation also results in a structural change where the figure-eight conformation changes to an open conformation. Energy optimization using the M062X/6-31G** level of DFT also indicates an open conformation upon protonation of 10. On the other hand, 12 remains nonaromatic in both freebase and protonated forms due to its rigidity with only four meso carbon bridges. The 48π decaphyrin 10 represents the largest decaphyrin reported to date.


 Please wait while we load your content...
Please wait while we load your content...