Conjugated oligomers with alternating heterocycles from a single monomer: synthesis and demonstration of electroluminescence†
Abstract
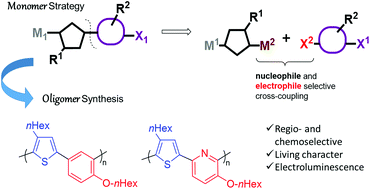
Conjugated oligomers based on two different heterocycles are typically prepared by step growth polycondensation cross-coupling methods from two monomers X-Cycle1-X and M-Cycle2-M with no control of the regioselectivity. In this work, we used a new synthetic strategy that involves an extremely chemoselective reaction of a dielectrophilic compound, X1-Cycle1-X2, with a dinucleophilic component, M1-Cycle2-M2, under Stille conditions. The resulting monomers, X1-Cycle1-Cycle2-M2 are di-heterocyclic push–pull monomers that still contain a nucleophilic site (boronic acid) and electrophilic site (bromide) and are set up for a controlled polymerization under Suzuki conditions. In this way, two semiconducting oligomers, based on thiophene/benzene and thiophene/pyridine motifs were synthesized. Both oligomers were characterized in terms of their thermal, electrochemical, absorption, emission and electroluminescence properties.


 Please wait while we load your content...
Please wait while we load your content...