Selectfluor™-catalyzed oxidative cyclization of ynamides enables facile synthesis of oxazolidine-2,4-diones†
Abstract
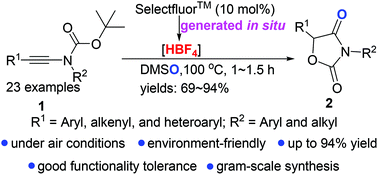
A Selectfluor™-catalyzed oxidative cyclization of ynamides affording oxazolidine-2,4-diones in yields from 69%–94% is described. The reaction is run under air and exhibits excellent chemo-selectivity and functional group compatibility. Preliminary mechanistic studies reveal that the solvent DMSO acts as an oxidant, and the redox reaction of Selectfluor™ with DMSO generates the real active catalyst HBF4in situ.


 Please wait while we load your content...
Please wait while we load your content...