Optimization of the synthesis of quinoline-based neutral cyclometalated iridium complexes via microwave irradiation: design of light harvesting and emitting complexes using bulky quinolines†
Abstract
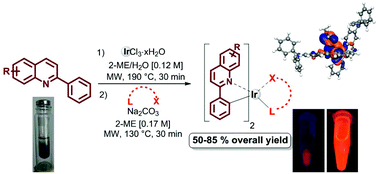
We report an optimized synthesis of neutral heteroleptic cyclometalated iridium complexes based on bidentate ligands (C^N) of 2-phenylpyridine and 2-phenylquinoline derivatives and an ancillary ligand (L^X) of acetylacetone via microwave (MW) irradiation. The developed methodology increases yields up to 85% with significantly lower reaction times and solvent consumption in comparison with conventional heating. The robustness of our methodology is demonstrated through the high-yield synthesis of bulky 2-phenylquinoline derivatives leading to three new complexes, which show desirable optical properties with absorption tails extending up to 650 nm and relative quantum yields of up to 0.210. In addition, the spin-allowed transitions for these complexes exhibit solvatochromism and are, therefore, characterized experimentally and computationally.


 Please wait while we load your content...
Please wait while we load your content...