Officinalins A and B, a pair of C23 terpenoid epimers with a tetracyclic 6/7/5/5 system from Salvia officinalis†
Abstract
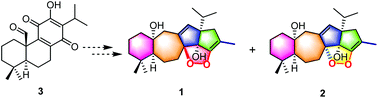
A pair of novel C23 terpenoid epimers, officinalins A (1) and B (2), together with a biogenetically related known diterpenoid (3), was isolated from the leaves of Salvia officinalis. The epimers feature an unprecedented carbon skeleton with a tetracycline-[9.6.0.03,8.012,16]-heptadecane core and a peroxide bridge. Their structures were unequivocally established by extensive spectroscopic analyses, including electronic circular dichroism (ECD) calculations and ROESY correlation analogy with model compounds for the configuration assignment. Possible biosynthetic pathways of 1 and 2 were proposed. Compound 1 exhibited potent NO inhibitory activity with an IC50 value of 2.02 ± 0.87 μM.


 Please wait while we load your content...
Please wait while we load your content...