Platinum(ii)-catalyzed dehydrative C3-benzylation of electron-deficient indoles with benzyl alcohols†
Abstract
A synthetic strategy for the water-promoted direct dehydrative coupling of indoles with benzyl alcohols catalyzed by PtCl2(PhCN)2 in 1,2-dichloroethane has been developed. Water molecules significantly accelerate C–C bond formation, whereas the reaction proceeds slowly under anhydrous conditions. Comparative rate plots for reactions in the presence of H2O and D2O show a kinetic solvent isotope effect (KSIE) of 1.5. A Hammett study of the dehydrative reaction of para-substituted benzhydrols shows negative ρ values, indicating a build-up of cationic charge during the rate-determining sp3 C–O bond cleavage step. Our catalytic system can be used on the gram scale with simplified product isolation and catalysis is performed using 1 mol% of a Pt(II) catalyst with an additional molecule of water.
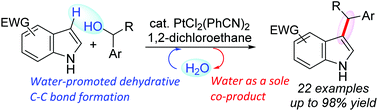


 Please wait while we load your content...
Please wait while we load your content...