Hydroxylammonium derivatives for selective active-site lysine modification in the anti-virulence bacterial target DHQ1 enzyme†
Abstract
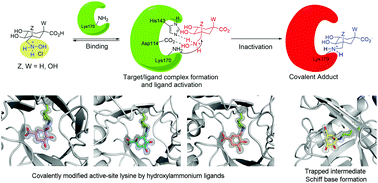
Targeted irreversible inhibitors bearing electrophiles that become activated towards covalent bond formation upon binding to a specific protein/enzyme is an emerging area in drug discovery. Targeting lysine residues is challenging due to the intrinsically low reactivity of the amino group at physiological pH. Herein we report the first example of a hydroxylammonium derivative that causes a specific covalent modification of an active-site and a sterically inaccessible lysine residue of an enzyme. The described ligands, compounds 1–3, were rationally designed to be activated towards covalent bond formation upon binding to the type I dehydroquinase (DHQ1) enzyme for the development of new anti-virulence agents to combat the widespread resistance to antibiotics. Evidence in atomic detail for the covalent modifications caused by the ligands to the catalytic Lys170 by the formation of a stable secondary amine is provided by the resolution at 1.08–1.25 Å of the crystal structures of DHQ1 from Salmonella typhi enzyme adducts. In addition, the first crystal structure of the addition intermediate adduct at 1.4 Å of a Schiff base formation reaction by using an analog of the natural substrate, compound 4, is also reported. Molecular dynamics simulation studies on non-covalent enzyme/ligand complexes and a two-dimensional QM/MM umbrella sampling simulation study suggested that a direct displacement by Lys170 with the release of NH2OH would be feasible. These studies might open up new opportunities for the development of novel lysine-targeted irreversible inhibitors bearing a methylhydroxylammonium moiety as a latent electrophile.
- This article is part of the themed collection: Recent Open Access Articles in Frontiers Journals


 Please wait while we load your content...
Please wait while we load your content...