Diastereoselective access to 2-aminoindanones via the rhodium(ii)-catalyzed tandem reaction involving O–H insertion and Michael addition†
Abstract
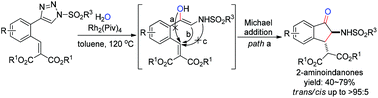
A rhodium(II)-catalyzed tandem reaction involving the O–H insertion of N-sulfonyl 1,2,3-triazoles with H2O and Michael addition has been developed for the construction of 2-amino-1-indanones by the formation of one new C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and one new C–C bond. The notable features of the strategy include acceptable to good yields, high diastereoselectivities, and mild conditions. It provides a good choice for the construction of libraries of highly functionalized indanone compounds.
O bond and one new C–C bond. The notable features of the strategy include acceptable to good yields, high diastereoselectivities, and mild conditions. It provides a good choice for the construction of libraries of highly functionalized indanone compounds.


 Please wait while we load your content...
Please wait while we load your content...