Transition metal-free, visible-light-mediated construction of α,β-diamino esters via decarboxylative radical addition at room temperature†
Abstract
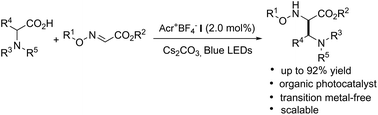
Transition-metal-free visible-light photoredox catalyzed decarboxylative radical addition has been developed for the construction of α,β-diamino esters from amino acids with glyoxylic oxime ethers under mild conditions. Cyclic and chain amino acids, dipeptides, and simple aliphatic acids are amenable to this addition as the alkyl radical source. A variety of α,β-diamino esters with broad functional groups were synthesized in satisfactory yields at room temperature. The employment of acridinium as the photocatalyst and its potential scalability also demonstrate the synthetic value in practice.
- This article is part of the themed collection: FOCUS: Radical-involved chemical transformations


 Please wait while we load your content...
Please wait while we load your content...