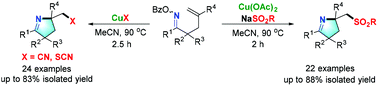
Iminyl radical-promoted imino sulfonylation, imino cyanogenation and imino thiocyanation of γ,δ-unsaturated oxime esters: synthesis of versatile functionalized pyrrolines†
Abstract
An efficient approach for the cascade radical cyclization/functionalization of γ,δ-unsaturated oxime esters was developed, which introduced useful functional groups (–SO2R, –CN, –SCN) into pyrrolines. Control experiments were conducted and a mechanism involving iminyl radical intermediates, which was initiated by Cu(I) species, was proposed.


 Please wait while we load your content...
Please wait while we load your content...