Why electrostatically enhanced thiourea is better than Schreiner's thiourea in both catalytic activity and regioselectivity?†
Abstract
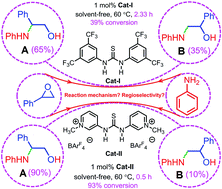
Electrostatically enhanced thiourea behaves more efficiently than Schreiner's thiourea in the ring-opening aminolysis of styrene oxide with aniline, and the underlying reasons were uncovered by DFT calculations, the distortion/interaction theory, and NBO and QTAIM analyses. Electrostatically enhanced thiourea combines with a substrate much stronger than Schreiner's thiourea, so it activates the substrate more efficiently and thus lowers the activation energy more significantly, leading to a higher reaction rate and a greater conversion.


 Please wait while we load your content...
Please wait while we load your content...