Aryldiazonium ion initiated C–N bond cleavage for the versatile, efficient and regioselective ring opening of aziridines†
Abstract
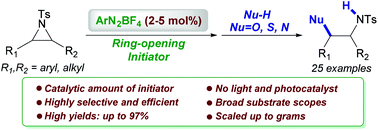
Aryldiazonium salts have been proved to initiate the regioselective cleavage of aziridines for the installation of varied functional groups. The ring-opening process is effective and general for a variety of nucleophiles, including [O], [S] and [N], at room temperature. This highly regioselective process, which can be performed at the gram scale, enjoys operational simplicity, as well as mild and metal-free conditions. The postulated reaction mechanism involves a single electron transfer from aziridines to aryldiazonium salts, which generates a highly reactive amino radical cation.


 Please wait while we load your content...
Please wait while we load your content...