Iron-mediated highly diastereoselective allylation of carbonyl compounds with cyclic allylic halides†
Abstract
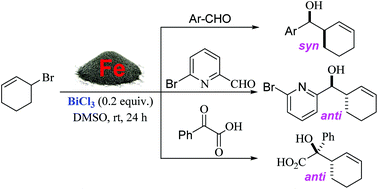
An efficient iron-mediated highly diastereoselective allylation of carbonyl compounds with cyclic allylic halides is reported. The allylation reactions involving various carbonyl compounds proceeded efficiently in the presence of 20 mol% bismuth(III) chloride to give the corresponding homoallylic alcohols in moderate to good yields with excellent diastereoselectivities and wide functional group tolerance. In cases where an aldehyde and a ketone (e.g., 2-pyridinecarboxaldehyde and phenylglyoxylic acid) containing an adjacent chelating atom were used, complete reversal of product diastereoselectivities was observed which could be explained by the Cram-chelated six-membered ring transition state.


 Please wait while we load your content...
Please wait while we load your content...