Highly selective hydrogenation of aldehydes promoted by a palladium-based catalyst and its application in equilibrium displacement in a one-enzyme procedure using ω-transaminase†
Abstract
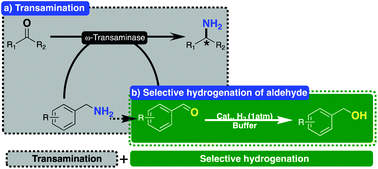
Presented here is a highly efficient procedure for the selective hydrogenation of aldehydes in the presence of ketones and its application in equilibrium displacement in a one-enzyme procedure catalysed by ω-transaminase. A palladium-based heterogeneous catalyst was chemically synthesized from K2PdCl4 and 1,1′-binaphthyl-2,2′-ylenediamine and found to be suitable for selective hydrogenation of aldehydes using H2 of 1 atm, with a wide spectrum of aldehydes being efficiently and selectively reduced to the corresponding alcohols with a selectivity of up to 99%. This catalyst and its developed procedure can be successfully applied in pushing the equilibrium to product formation during transamination in a ω-transaminase-promoted one-enzyme procedure via hydrogenation of the co-product, and an array of chiral amines was afforded in good to excellent yields of up to 99% with ees of up to >99%.


 Please wait while we load your content...
Please wait while we load your content...