Copper-catalyzed versatile C(sp3)–H arylation: synthetic scope and regioselectivity investigations†
Abstract
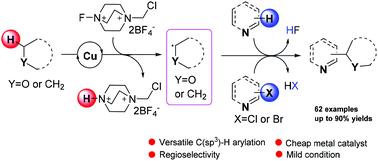
The copper-catalyzed versatile C(sp2)–C(sp3) bond formation with N-heteroaromatics and hydrogen donors was developed. Various alkanes and ethers reacted with quinolines, isoquinolins, pyridines, benzooxazole and benzothiazole to give the corresponding C(sp2)–H alkylation products via cross-dehydrogenative coupling. On the other hand, we achieved the high regioselective C(sp2)–halogen alkylation of (hetero)aryl chlorides and (hetero)aryl bromides with ethers via elimination of the halogen radical. The reaction mechanism was investigated with control experiments.


 Please wait while we load your content...
Please wait while we load your content...