Highly efficient biocatalytic desymmetrization of meso carbocyclic 1,3-dicarboxamides: a versatile route for enantiopure 1,3-disubstituted cyclohexanes and cyclopentanes†
Abstract
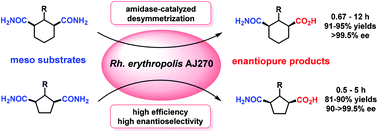
A versatile biocatalytic desymmetric strategy for efficiently accessing enantiopure 1,3-disubstituted cyclohexanes and cyclopentanes was developed. Desymmetric hydrolysis of a series of meso carbocyclic 1,3-dicarboxamides catalyzed by Rhodococcus erythropolis AJ270 whole cells under mild conditions afforded 3-carbamoylcyclic carboxylic acid derivatives in 81–95% yields and >99.5% ee values. The desymmetrization of different types of substrates shows a general S-selectivity, and the catalytic efficiency and enantioselectivity show dependence on the cyclic structure of the substrates. The biocatalytic products were conveniently transformed to chiral bicyclic oxazolidinone compounds.


 Please wait while we load your content...
Please wait while we load your content...