Cobalt-catalyzed direct transformation of aldehydes to esters: the crucial role of an enone as a mediator†
Abstract
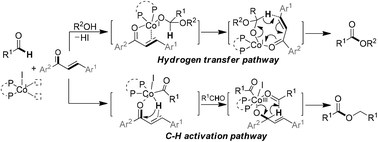
An oxidative esterification of aldehydes with alkanols catalyzed by an in situ generated low-valent cobalt system has been developed using an enone as a mild oxidant. Mechanistic studies revealed that it proceeds through a Co(I)-catalyzed hydrogen-transfer route, wherein the α-vinyl moiety in the bidentate enone functions as a hydride acceptor. Meanwhile, Co(I)-catalyzed formyl C–H activation occurred as a competing reaction leading to aldehyde dimerization. The occurrence of the usually kinetically disfavored hydride transfer step therein was significantly increased in the presence of an enone reacting as a hydride transfer initiator.


 Please wait while we load your content...
Please wait while we load your content...