A unified and straightforward total synthesis of (+)-porantheridine and (−)-6-epi-porantheridine†
Abstract
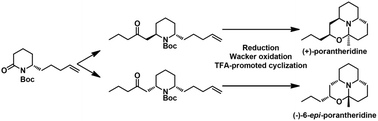
A unified asymmetric total synthesis of (+)-porantheridine and (−)-6-epi-porantheridine has been accomplished. The two molecules were prepared via a Horner–Wadsworth–Emmons/aza-Michael addition reaction or diastereoselective nucleophilic substitution of 2-methoxypiperidine from a common intermediate, respectively. The key features of the unified synthetic strategy also included an asymmetric addition of silyl dienolate to sulfinylimine, substrate-controlled reduction, Wacker oxidation and TFA-promoted cyclization.


 Please wait while we load your content...
Please wait while we load your content...