Bifunctional oxindole-chromone 4C building block directed asymmetric synthesis of bispirocyclic hexahydroxanthones featuring five contiguous stereocenters and two side-by-side oxindoles†
Abstract
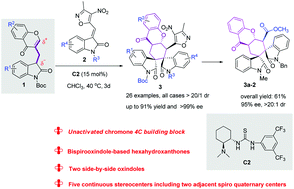
Optically active small molecules based on privileged natural product frameworks and rich in three-dimensional complexity are in high demand. In this context, the merger of two bispirooxindole and hexahydroxanthone cores is a powerful and routine strategy to develop bioactive small molecules. Here, we wish to report the bifunctional oxindole-chromone 4C building block 1 directed asymmetric synthesis of a new class of potential biological structurally bispirooxindole-based hexahydroxant-hones 3 with five adjacent stereocentres, of which two are adjacent spiro quaternary stereocentres. All the products 3 are smoothly obtained with excellent results (up to 91% yield, >20 : 1 dr and up to >99% ee). It is noteworthy that such a protocol represents a novel organocatalytic approach for the synthesis of highly desirable but challenging hexahydroxanthone derivatives, which will aid the search for new bioactive molecules.


 Please wait while we load your content...
Please wait while we load your content...