A concise approach to the tricyclic framework of longipinane- and ent-longipinane-type sesquiterpenoids†
Abstract
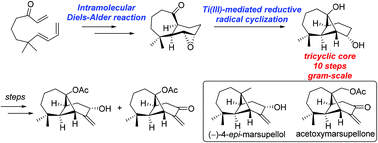
A concise and efficient strategy for the synthesis of the common 6,6-dimethyltricyclic[5.4.0.02,8]undecane framework of longipinane- and ent-longipinane-type sesquiterpenoids has been developed. This approach features an intramolecular Diels–Alder reaction to construct the 6/7 cis-fused ring system and a Ti(III)-mediated reductive radical cyclization reaction to access the highly strained bridged cyclobutane moiety.


 Please wait while we load your content...
Please wait while we load your content...