Intermolecular oxidative radical fluoroalkylfluorosulfonylation of unactivated alkenes with (fluoroalkyl)trimethylsilane, silver fluoride, sulfur dioxide and N-fluorobenzenesulfonimide†
Abstract
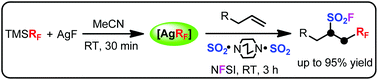
An intermolecular oxidative radical fluoroalkylfluorosulfonylation reaction of unactivated alkenes with convenient and commercially available (fluoroalkyl)trimethylsilane, silver fluoride, sulfur dioxide and N-fluorobenzenesulfonimide (NFSI) is described. This transformation efficiently affords various fluoroalkyl-containing alkyl sulfonyl fluorides with good functional group tolerance under mild conditions. Silver fluoroalkyl complexes as the fluoroalkyl radical source generated from (fluoroalkyl)trimethylsilane and silver fluoride may be the key intermediate.
- This article is part of the themed collection: FOCUS: Radical-involved chemical transformations


 Please wait while we load your content...
Please wait while we load your content...