Organocatalytic atropo- and E/Z-selective Michael addition reaction of ynones with α-amido sulfones as sulfone-type nucleophile†
Abstract
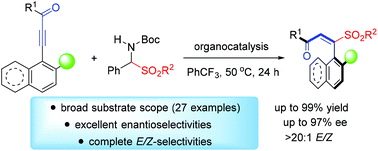
We describe herein the first enantioselective organocatalytic Michael addition reaction of α-amido sulfones to ynones to access axially chiral styrenes. Sulfone-containing axially chiral styrene compounds were constructed with excellent enantioselectivity and almost complete E/Z-selectivity (up to 97% ee, >20 : 1 E/Z) in the presence of an easily accessible cinchona-alkaloid-derived multiple hydrogen-bonding catalyst. The enantioenriched sulfone-containing products were used as precursors for further transformations without any loss of the ee value. The equilibration process of this transformation was monitored by 1H NMR spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...