N-Heterocyclic carbene catalyzed chemo- and enantioselective cross-benzoin reaction of aldehydes with isatins†
Abstract
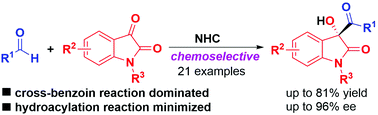
A highly chemoselective intermolecular cross-benzoin reaction of aldehydes with isatins enabled by asymmetric N-heterocyclic carbene (NHC) catalysis is described, and it provides 3-substituted-3-hydroxyoxindoles in moderate to good yields with moderate to excellent enantioselectivities. This reaction can be readily scaled up, the products could be straightforwardly reduced to the corresponding 1,2-diols, and the trityl (Trt) protecting group could be easily removed without erosion of enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...