Structural assignment of the enol–keto tautomers of one-pot synthesized 4-hydroxyquinolines/4-quinolones†
Abstract
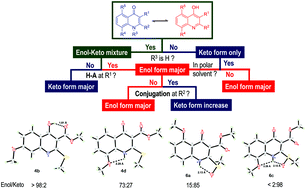
The one-pot preparation of 2,3-disubstituted 4-quinolones and the structural assignment of their tautomers are described. The mono-selective Michael addition of anilines to α,α-dioxoketene dithioacetals followed by thermal cyclization of the crude N,S-acetals gave the desired 4-hydroxyquinolines in good to excellent yields. The tautomeric structures of the obtained products were confirmed by X-ray crystallography, IR, and NMR experiments. Spectroscopic data revealed that the equilibrium between the enol and keto forms of the bicyclic system was determined by the strength of the internal H-bonds. A H-bond acceptor at the 3-position favored the enol form via 6-membered intramolecular H-bonding. A H-bond acceptor at the 2- or 8-position completely switched the equilibrium to favor the keto form possibly due to extended conjugation and H-bonding. The experimental assignments were perfectly matched with the results of DFT calculation.


 Please wait while we load your content...
Please wait while we load your content...