Synthesis and thermodynamic investigation of MnO nanoparticle anchored N-doped porous carbon as the anode for Li-ion and Na-ion batteries†
Abstract
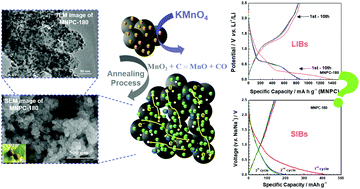
A platanus-fruit-like manganese monoxide (MnO)/nitrogen-doped porous carbon (MNPC) is prepared by a simple and low-cost method, in which MnO nanoparticles are combined with the polypyrrole-based hydrogel (PPH) derived nitrogen-doped porous carbon (NPC). When used as an electrode, the porous NPC acts as an effective support for the MnO nanoparticles that can provide a high ion storage capacity. Consequently, MNPC shows a good cycling stability and an enhanced rate capability in lithium-ion batteries, and the composite performs better than NPC and bare MnO particles alone. However, in sodium-ion batteries, MNPC exhibits a similar electrochemical behavior as NPC, indicating that MnO nanoparticles are much less active for storing sodium. This is likely because the thermodynamic driving force and redox potential for sodium storage in MnO are much lower than those for lithium storage. Thus, this study helps to thermodynamically reveal the lithiation and sodiation properties of MnO materials.


 Please wait while we load your content...
Please wait while we load your content...