Fluoride-ion solvation in non-aqueous electrolyte solutions†
Abstract
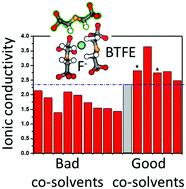
Understanding the factors that influence ion-solvent properties for the fluoride ion in organic solvents is key to the development of useful liquid electrolytes for fluoride-ion batteries. Using both experimental and computational methods, we examined a range of chemical and electrochemical properties for a set of organic solvents in combination with dry N,N,N-trimethylneopentylammonium fluoride (Np1F) salt. Results showed that solvent electronic structure strongly influences Np1F dissolution, and the pKa of solvent protons provides a good guide to potential F− reactivity. We found a number of organic solvents capable of dissolving Np1F while providing chemically-stable F− in solution and characterized three of them in detail: propionitrile (PN), 2,6-difluoropyridine (2,6-DFP), and bis(2,2,2-trifluoroethyl) ether (BTFE). Arrhenius analysis for Np1F/PN, Np1F/DFP, and Np1F/BTFE electrolytes suggests that DFP facilitates the highest F− ion mobility of the three neat solvents. Electrolyte mixtures of BTFE and amide co-solvents exhibit higher ionic conductivity than the neat solvents. This improved ionic conductivity is attributed to the ability of BTFE:co-solvent mixtures to partition between Np1+ and F− ion-aggregates, promoting better ion dissociation.
- This article is part of the themed collection: In Honour of Professor Robert H. Grubbs for His 50-year Contributions in Metathesis


 Please wait while we load your content...
Please wait while we load your content...