Synthesis of fused heterocyclic systems via the Mallory photoreaction of arylthienylethenes†
Abstract
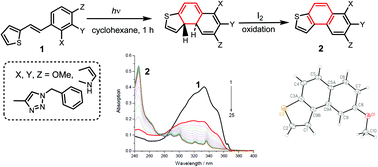
Photochemical oxidative cyclization of 2- and 3-thienylstilbenes (heterostilbenes) containing mono-, di- and trimethoxy groups in the benzene ring or heterocyclic fragment results in the formation of isomeric thieno-annelated polycyclic aromatic compounds demonstrating optical properties that differ from those of initial stilbene derivatives. The structures of cyclic products were evaluated via1H and 13C NMR, HRMS, elemental analysis and X-ray crystallography. The research was aimed to study the effect of substituents in stilbene derivatives of thiophene as well as the position of the styryl fragment in the thiophene nucleus on the occurrence of photocyclization reactions.


 Please wait while we load your content...
Please wait while we load your content...