The detection of the precursors of the photorearranged products of 3-hydroxyflavones in selected solvents from UV-visible spectra in situ†
Abstract
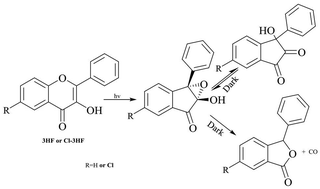
Mechanistic studies relating to the photochemistry of 3-hydroxy-2-phenyl-4H-chromen-4-one (3HF) and 6-chloro-3-hydroxy-2-phenyl-4H-chromen-4-one (Cl-3HF) have been reinvestigated in selected solvents. The UV-visible spectra of the photoproduct(s) of 3HF and Cl-3HF have been computed in situ via subtracting the spectra of unreacted substrates, with acetonitrile (ACN) and methanol (MeOH) as solvents. These spectra turn out to be different from the spectra of the corresponding isolated photoproducts: 3-hydroxy-3-phenyl-indan-1,2-dione and 6-chloro-3-hydroxy-3-phenyl-indan-1,2-dione (referred to as dione ). Analyses of the photoproduct(s) via GC-MS show the formation of a single detectable product, i.e., the corresponding dione. On the basis of some experimental observations, it is proposed that the primary photoproduct in situ is 2,3-epoxy-2-hydroxy-1-indanone (referred to as epoxide) instead of dione as reported in previous years. Earlier, epoxide has been proposed to be the intermediate in the mechanism for the formation of dione. This is the first report where the formation of epoxide has been directly detected in the selected solvents. On the other hand, both dione and epoxide (2 : 1) are shown to be formed with MeOH as solvent. The second important finding is that epoxide and dione interconvert in the dark, depending upon the environment. With ACN as solvent, pure dione in the dark is kinetically and partially converted to epoxide. With MeOH as solvent, epoxide is instantly and partially converted to dione until both are in equilibrium. However, a solution of dione in MeOH remains stable in the dark. The photoformation of epoxide is quantitative with ACN as solvent and it is sufficiently stable. It has been further observed that epoxide solutions of 3HF and Cl-3HF in ACN are quantitatively converted into 3-phenylisobenzofuran-1(3H)-one and 6-chloro-3-phenylisobenzofuran-1(3H)-one, i.e., the corresponding phthalides, through the loss of CO when kept in the dark for some days. A mechanism has been proposed where epoxide has been shown to give dione and/or phthalide via selective C–O or C–C bond cleavage in the oxiranyl ring, respectively. The selection of this cleavage depends mainly on the solvent system and the substituents in the parent flavones.


 Please wait while we load your content...
Please wait while we load your content...