Excited-state intramolecular proton transfer in a bioactive flavonoid provides fluorescence observables for recognizing its engagement with target proteins†
Abstract
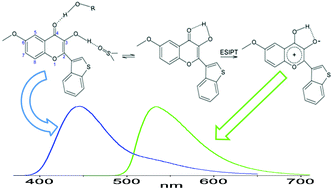
A benzothiophene-substituted chromenone with promising activity against Leishmania and Trypanosoma species exhibits peculiar fluorescence properties useful for identifying its complexes with target proteins in the microorganism proteomes. The emission spectra, anisotropy and time profiles of this flavonoid strongly change when moving from the free to the protein-bound forms. The same two types of emission are observed in organic solvents and their mixtures with water, with the relative band intensities depending on the solvent ability to establish hydrogen bonds with the solute. The regular emission prevails in protic solvents, while in aprotic solvents the anomalously red-shifted emission occurs from a zwitterionic tautomeric form, produced in the excited state by proton transfer within the intramolecularly H-bonded form. This interpretation finds support from an experimental and theoretical investigation of the conformational preferences of this compound in the ground and lowest excited state, with a focus on the relative twisting about the chromenone–benzothiophene interconnecting bond. An analysis of the absorption and emission spectra and of the photophysical properties of the two emitting tautomers highlights the relevance of the local microenvironment, particularly of the intra- and intermolecular hydrogen bonds in which this bioactive compound is involved, in determining both its steady-state and time-resolved fluorescence behaviour.
- This article is part of the themed collection: In memory of Professor Ugo Mazzucato (1929–2017)


 Please wait while we load your content...
Please wait while we load your content...