A visible chemosensor based on carbohydrazide for Fe(ii), Co(ii) and Cu(ii) in aqueous solution†
Abstract
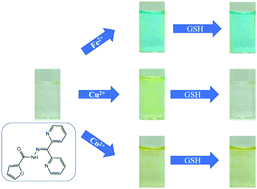
A colorimetric sensor with pyridyl and carbohydrazide components has been synthesized and visibly turns blue in the presence of Fe(II). The colorless sensor also changes color when exposed to Co(II) and Cu(II), but its color becomes yellow. The sensor shows no visible response to other metal ions such Ca2+, Cr3+, Mn2+, Fe3+, Ni2+, Zn2+, Cd2+, Ag+, Hg2+, and Pb2+. The binding ratio of the sensor to Fe(II), Co(II), and Cu(II) is 2 sensors to 1 metal ion. The binding constants of the sensor are: Fe(II): 1.0 × 109 M−2, Co(II): 2 × 109 M−2, and Cu(II): 3.0 × 109 M−2. The sensor works well at neutral pH and micromolar concentrations of Fe(II), Co(II), and Cu(II) can be detected in water samples. The sensor's color response to Cu(II) is uniquely attenuated by glutathione.


 Please wait while we load your content...
Please wait while we load your content...