The dephosphorylated S8A and S18A mutants of (oat) phytochrome A comprise its two species, phyA′ and phyA′′, suggesting that autophosphorylation at these sites is not involved in the phyA differentiation
Abstract
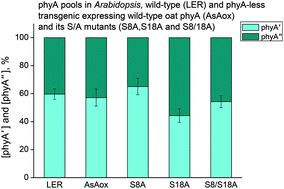
Phytochrome A (phyA) is represented in plants by two species, phyA′ and phyA′′, with different properties and modes of action (Sineshchekov, Funct. Plant Biol., 2019, 46, 118–135). They differ by the modification of a serine(s) residue at the N-terminus, possibly, by phosphorylation. To verify if these serines could be the Ser8 and Ser18 (in Avena sativa phyA, AsphyA), whose autophosphorylation modulates AsphyA stability and sensitivity as shown with the use of the serine-to-alanine substitution AsphyA mutants (S8A, S18A and S8/18A) (Han et al., Plant Cell Physiol., 2010, 51, 596–609), we have undertaken low-temperature (85 K) fluorescence investigations of phyA in these transgenic lines. The content and proportion of phyA′ and phyA′′ were essentially the same in wild-type AsphyA and its mutants, and in endogenous Arabidopsis phyA. All the lines revealed a higher phyA′/phyA′′ proportion upon longer germination-inducing preillumination (3 h vs. 15 min white light) supporting our earlier finding that the phyA differentiation into the subpools is light-regulated. These observations and our earlier data imply that this process involves N-terminal serine(s) different from the autophosphorylated Ser8 and Ser18 (in AsphyA) narrowing down the area of further search for the exact site(s) of the phyA modification.


 Please wait while we load your content...
Please wait while we load your content...