Facile synthesis of 1,2,4,5-tetrahydro-1,4-benzodiazepin-3-ones via cyclization of N-alkoxy α-halogenoacetamides with N-(2-chloromethyl)aryl amides†
Abstract
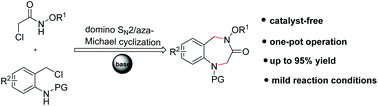
A facile and efficient cyclization of N-alkoxy α-halogenoacetamides with N-(2-chloromethyl)aryl amides has been achieved for rapid access to 1,2,4,5-tetrahydro-1,4-benzodiazepine-3-one derivatives (up to 95% yield). The intriguing features of this intermolecular cyclization include its mild reaction conditions and easy handling for scalable synthesis.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...