Synthesis and biological profiling of parthenolide ether analogs†
Abstract
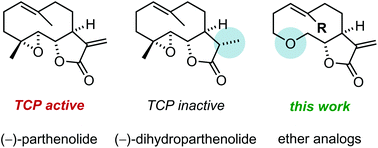
Parthenolide (PTL) strongly inhibits the detyrosination of microtubules and accelerates neuronal growth. In order to access cyclic ether derivatives of PTL, ring-closing metathesis (RCM) was investigated in comparison to intramolecular sulfone alkylation. Incompatibility of RCM with epoxides was found in this setting. Biological evaluation for tubulin carboxypeptidase inhibition indicated that the epoxide is crucial for parthenolide's activity.
- This article is part of the themed collections: Chemical Biology in OBC and Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...